Which of the Lewis structure is best at approximating the real situation becomes secondary, and it makes sense to move on from a Lewis structure model to more comprehensive models of bonding for species such as this one Share Improve this answer Follow answered Nov 23 '19 at 2236 KarstenG L 477 mN m 21 at 1 8CAmmonium ion, NH 4 amide ion, NH 2formaldehyde, HCOH;

Meso Hydrobenzoin 99 579 43 1 Sigma Aldrich
1 1-ethanediol lewis structure
1 1-ethanediol lewis structure-Quantum chem calcns were carried out to study the gasphase structure, stability, and decompn of the two simplest alkanediols, methanediol and 1,1ethanediol, in the presence of various catalysts Three different conformers for monomeric alkanediols cis, trans, and trans' were consideredFrom the video Drawing Lewis Structures An Example 1 Draw the Lewis structure of SF 2 From the video Lewis Structures Further Examples, draw the Lewis structures of 1 C 2 H 2 2 ClOR ESONANCE AND F ORMAL C HARGES From the video Resonance in Lewis Theory 1



Experiment W12 Organic Structures And Molecular Models 7 Build Models Of All Possible Isomers Of Chcl Homeworklib
In TiBeta (), we see differences in TiO distances ranging from 1787 to 1795 ÅThese differences are due to the different local flexibility of the framework at the different Tsites The TiO distances in TS1 range from 17 to 17 ÅThe average values for each structure show the same values for the TiO distances, 179 Å, in agreement with EXAFS measurementsQuantum chemical calculations have been carried out to investigate the gasphase structure, stability, and decomposition of the two simplest alkanediols, methanediol and 1,1ethanediol, in theSource(s) lewis dot structure asf6 1 https//trim/iUDJC 0 0 Corin 9 years ago The above answer is correct, but you don't need the negatives on anything The As in the middle with 6 F's around it, each with 6 dots (3 pairs of 2) is enough Source(s) Chemistry student now 0 1
Chemical structure This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or Javascript Species with the same structure Pluronic f68CH 2 CF 2 );The Lewis structures for F 2 and O 2 are written as follows The prefix "co" is used to indicate that two or more entities are joined or have equal standing (as in, for example, coexist, cooperate, and coordinate ) It is therefore appropriate that the term "covalent bond" is used to describe molecular bonds that result from the sharing of one
305 (t, J 215 Hz;The diamagnetic susceptibility was determined using Gouy's method The diamagnetic susceptibilities of 1,1ethanediol and 1,1propanediol were −391 × 10 −6 cm 3 mol −1 and −515 × 10 −6 cm 3 mol −1 at 287 K, respectivelySearch results for PHRG at SigmaAldrich Compare Products Select up to 4 products *Please select more than one item to compare


Rcsb Pdb 5o7w Crystal Structure Of The 4 Fluoro Rsl Lectin In Complex With Lewis X Tetrasaccharide



Oneclass C Does The Molecule Have Only One Unique Shape In 3 D Hint How Are The Hydrogen Atoms O
The absorbance at 565 nm is also used to measure cell density Detection limits are ~5 mg L1 for glucose, 50 mg L1 for lactic acid and 02 g L1 for proteins (rectilinear calibration ranges 5 mg L1 to 2 g l1, 01 to 2 g L1 and 02 to 8 g l1, respectively) The apparatus is particularly suitable for studies of fermentation kineticsEthylene glycol is a 1,2glycol compound produced via reaction of ethylene oxide with waterIt has a role as a metabolite, a toxin, a solvent and a mouse metabolite It is a glycol and an ethanediolSearch results for DMAEMA at SigmaAldrich Compare Products Select up to 4 products *Please select more than one item to compare


1 1 Ethanediol C2h6o2 Pubchem



Oneclass C Does The Molecule Have Only One Unique Shape In 3 D Hint How Are The Hydrogen Atoms O
The diamagnetic susceptibility was determined using Gouy's method The diamagnetic susceptibilities of 1,1ethanediol and 1,1propanediol were −391 × 10 −6 cm 3 mol −1 and −515 × 10 −6 cm 3 mol −1 at 287 K, respectivelyAcetate ion, CH 3 COOmethyl amine, CH 3 NH 2;CAS Registry Number ;



Cas 302 17 0 Chloral Hydrate Lookchem



Openstax General Chemistry Ch Organic Chemistry Top Hat
The structural formula is given in section 7 The CAS name is 2,2,2trichloro1,1ethanediol Synonyms include chloral monohydrate, trichloroacetaldehyde hydrate, trichloroacetaldehyde monohydrate, and 1,1,1trichloro2,2dihydroxyethane Centrosome structure and function is altered by chloral hydrate and diazepam during the firstLewis Structure Worksheet #2 – Page 2 15 Draw two resonance structures for the formate ion, CHO 2 and calculate the C—O bond order in the molecule 16 Draw three possible Lewis structures for the cyanate ion, CNO , where C is the centralStructure, properties, spectra, suppliers and links for 1,1Ethanediol,


Organic



Sch4u Exam Review Questions Glebe
A Lewis structure is based on the concept of the octet rule, in which atoms share electrons so that each atom has eight electrons in its outer shell As an example, an oxygen atom has six electrons in its outer shell In a Lewis structure, these six dots are arranged so that an atom has two lone pairs and two single electrons304 (t, J 215 Hz;O 3 lewis structure In the lewis structure of NH 3, you can see there are one double bond and one single bond between oxygen atoms From three oxygen atoms one oxygen atom has one lone pair Another one has two lone pairs and remaining one has three lone pairs Also, one oxygen atom has a 1 charge and another oxygen atom has a 1 charge



Ch3ch2oh Lewis Structure How To Draw The Lewis Structure For Ch3ch2oh Youtube


Ethylene Glycol Molecule Of The Month June 18 Jsmol Version
Drawing the Lewis Structure for I 3Video Drawing the Lewis Structure for I 3For the I3 Lewis structure we first count the valence electrons for the I3 molecule using the periodic table Once we know how many valence electrons there are in I3 we can distribute them around the central atom and attempt to fill the outer shells of each atomLewis Structure Worksheet 1 Answer Key Along with Lewis Dot Diagram Worksheet Answers Awesome Electron Dot Diagrams Finally, you will be able to review your materials list as well as the assignment by completing these extra materials By doing so, you will be able to review and understand how to do each task on your list and then make sureDiethylether, CH 3 CH


Ethylene Glycol Molecule Of The Month June 18 Jsmol Version


1 2 Ethanediol 1 2 14c2 C2h6o2 Pubchem
Its Lewis structure would just be 'Li' with no dots around it When electrons are gained, a negative ion (known as an anion) is formed Chlorine has 7 valence electrons and gains 1 electron during ionization, giving it a full shell of 8 electrons Its Lewis structure would be 'Cl' with 4 pairs of dots around itIn the Lewis structures listed here, M and X represent various elements in the third period of the periodic table Write the formula of each compound using the chemical symbols of each element (a) (b) (c) (d) Write the Lewis structure for the diatomic molecule P 2, an unstable form of phosphorus found in hightemperature phosphorus vaporQuestion Use This Condensed Chemical Structure To Complete The Table Below CH CHC CH The Condensed Chemical Structure Of Isobutene Some Facts About The Isobutene Molecule Number Of Carboncarbon Single (CC) Bonds Number Of Carbonhydrogen Single (CH)bonds Number Of Carboncarbon Double (C C) Bonds Decide Whether The Lewis Structure Proposed For Each



3 3 Dimethyl 2 Butanone 75 97 8 C6h12o Density Melting Point Boiling Point Structural Formula Synthesis



Experiment W12 Organic Structures And Molecular Models 7 Build Models Of All Possible Isomers Of Chcl Homeworklib
At low temperatures and low water partial pressures, amorphous deposits of molecular H(2)SO(4) complexed with variable amounts of H(2)O in a ratio of between 11 and 21 are formedVideo 11 Please look at Handout 1 Lewis dot structure technique while watching this videoNote steps 1 and 2 are switched between the video and the handout, but all the rest are the same Question Why does sulfur trioxide require resonance structures?In your answer, think about how a Lewis dot structure represents a bond, and the nature of PI bondsDraw the Lewis structure for 1 ethanol (CH 3 CH 2 OH) 2 bromoamine (NH 2 Br) 3 fluoroethyne (C 2 HF) 4 ethanol (C 2 H 5 OH) 5 dichlorodiiodomethane (CCl 2 I 2) 6 nitrogen (N 2) 7 ethanethiol (CH 3 CH 2 SH) Expert Answer 100% (14 ratings) Previous question Next question


1 2 Ethanediol Hoch2ch2oh Pubchem



Molecule Gallery Alcohols
Lewis dot structures are useful to predict the geometry of a molecule Sometimes, one of the atoms in the molecule does not follow the octet rule for arranging electron pairs around an atom This example uses the steps outlined in How to Draw A Lewis Structure to draw a Lewis structure of a molecule where one atom is an exception to the octet ruleCH 2 CF 2 ) at 1875 8C, literature value 24± 27 7305 mN m 21 at 18 8C), diiodomethane (99%;14" ElectronDot Model of Bonding Lewis Structures" Lewis structures are drawn by following simple rules 1 Draw the molecular skeleton 2 Count the number of available valence electrons • Add one electron for each negative charge, if an anion • Subtract one electron for each positive charge, if a cation 3


Staggered Vs Eclipsed Conformations Of Ethane Master Organic Chem



See The Appendix For Information On How To Accurately Weigh Samples Using Weigh Course Hero
An outline of how to detemine the "best" Lewis structure for an example, NO 3is given below 1 Determine the total number of valence electrons in a molecule 2 Draw a skeleton for the molecule which connects all atoms using only single bonds In simple molecules, the atom with the most available sites for bondng is usually placed centralComplete the questions below, showing the transfer of electrons between metal and nonmetal, the Lewis structure of the resulting compound, the formula unit, and the name of the compound (remember the anion name ends with –ide) Using dot diagrams, show the transfer ofIn the PdIII system, the molar absorptivity is 34 x 10^4 L mol1 cm1 at 630 nm and Beer's law is obeyed in the range 15 µg Pd mL1 Coexisting noble metal ions do not interfere An optical fiber detection cell for flow injection analysis and an optical fiberspectrophotometer were designed and constructed in house
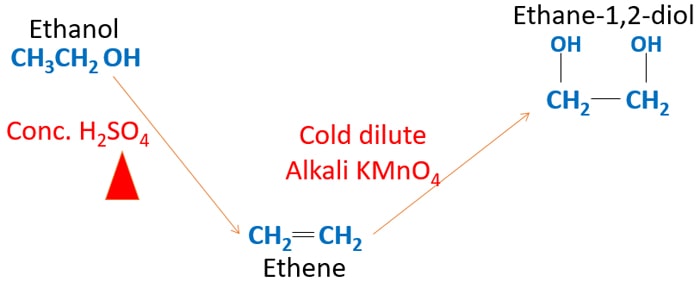


Ethanol To Ethane 1 2 Diol Ethylene Glycol 1 2 Ethanediol



Organic Chemistry I Homework 1998
The condensed chemical structure of 1,1ethanediol Some facts about the 1,1ethanediol molecule number of carboncarbon single ( ) bonds number of carbonhydrogen single ( ) bonds number of oxygenhydrogen single ( ) bonds number of lone pairs Chemists often draw the Lewis structure of organic compounds like 1,1ethanediol in a " condensed " style normal condensed In the condensed style, many of the groups in a molecule may be replaced by their chemical formulasTable 11 Relative permittivity and boiling point under atmospheric pressure of some polyols comparison with water and monoalcohols Water 1,2 1,2 Di(ethylene Methanol Ethanol 1ethanediol propanediol glycol) octanol er 785 38 32 32 33 24 10 Tb (C) 100 198 1 245 65 785 194374 (±CH 2 ±COO);
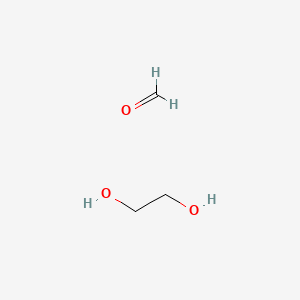


Ethane 1 2 Diol Formaldehyde C3h8o3 Pubchem


Staggered Vs Eclipsed Conformations Of Ethane Master Organic Chem
Combine H 16 and F to give the Lewis structure for hydrogen fluoride H F We are told that C2H6 has a carbon–carbon bond Thus, we combine two C and six H to write the HH Lewis structure H C C H HH of ethane There are a total of 14 valence electrons distributed as shown Each carbon is surrounded by eight electrons 17 (b) Each carbon1,1Ethanediol C2H6O2 CID structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safetyA stepbystep explanation of how to draw the NO3 Lewis Structure (Nitrate Ion) Get more chemistry help at wwwBreslynorgFor the NO3 Lewis structure,



See The Appendix For Information On How To Accurately Weigh Samples Using Weigh Course Hero
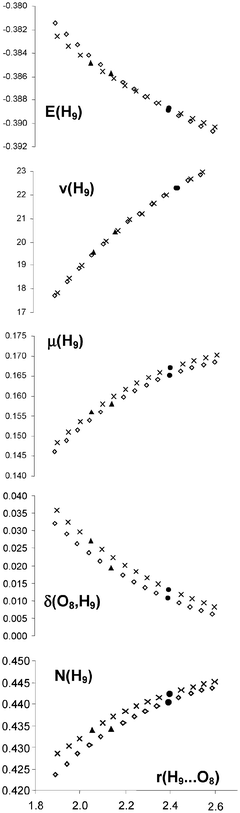


Do 1 2 Ethanediol And 1 2 Dihydroxybenzene Present Intramolecular Hydrogen Bond Physical Chemistry Chemical Physics Rsc Publishing
1,1Ethanediol acetic acid (12) Molecular Formula C 6 H 14 O 6;CHM 4 — House L1 Drawing Lewis Structures (Text ) Name 1 Below is a summary procedure of drawing Lewis dot structures for molecules A Sum the number of valence (outer) electrons for the entire molecule Remember the number of valence electrons for an Agroup element is given by the number of the group BExercise 13 Draw Lewis structures for the following species (use lines to denote bonds, dots for lonepair electrons) All atoms should have a complete valence shell of electrons ammonia, NH 3;


Illustrated Glossary Of Organic Chemistry Ethylene Glycol
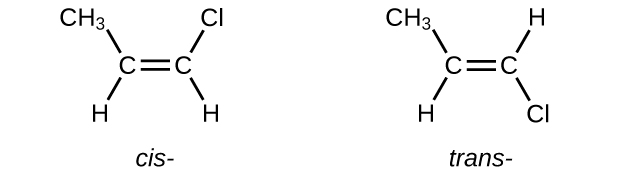


E Organic Chemistry Exercises Chemistry Libretexts
1 Complete the Lewis dot structure(s) for 2 Complete the Lewis dot sttllcture(s for 3 Complete the Lewis dot structure(s) for COC12 4 Complete the Lewis dot structure(s) for NOY 8Në 5, Complete the Lewis dot structure(s) for C103 STOP Your group will check your answers with the instructor before moving on Model VI MultiCentered MoleculesLewis Dot Structures can be produced by following a sequence of steps Let's produce a Lewis Dot Structure for NH 4 (the ammonium ion) Step 1 Count valence electrons N = 5 4 x H = 4 x 1 = 4 "" = 1 Total = 541= 8 electrons = 4 bonds and lone pairs Step 2!Arrange the atoms (identify a central atom, if possible)Monoisotopic mass Da;



Molecule Gallery Alcohols



Ethylene Oxide Wikipedia
G L 487 mN m 21 at 1 8C, literature value 24± 27 5076 mN m 21 at 8C) and 1,1ethanediol (ethylene glycol, 99%;O 3 lewis structure In the lewis structure of NH 3, you can see there are one double bond and one single bond between oxygen atoms From three oxygen atoms one oxygen atom has one lone pair Another one has two lone pairs and remaining one has three lone pairs Also, one oxygen atom has a 1 charge and another oxygen atom has a 1 chargeA stepbystep explanation of how to draw the PF4 Lewis Structure For the PF4 Lewis structure use the periodic table to find the total number of valenc



Solved Tan Models Are Useful For Visai A Tot Models Le Lo Chegg Com



4 Build A Model Of The 1 2 Ethanediol Molecule A Identify The Electron Pair And Molecular Geometry Around Homeworklib
Draw the Lewis structure for 1 ethanol (CH 3 CH 2 OH) 2 bromoamine (NH 2 Br) 3 fluoroethyne (C 2 HF) 4 ethanol (C 2 H 5 OH) 5 dichlorodiiodomethane (CCl 2 I 2) 6 nitrogen (N 2) 7 ethanethiol (CH 3 CH 2 SH) Expert Answer 100% (14 ratings) Previous question Next questionAverage mass 1172 Da;1 A COMPREHENSIVE GUIDE TO THE Amines 264 142 Hydrogen Cyanide 319 143 Sodium Cyanide 322 Chapter 10 Asbestos 269 144 Potassium Cyanide 324 101 Structure and 145 Calcium Cyanide 325 Properties 269 146 Cyanogen 326 102 Uses and Exposure 147 Cyanogen Chloride 327 Risk 270 148 Cyanogen Bromide 328 103 Health Hazard and 14
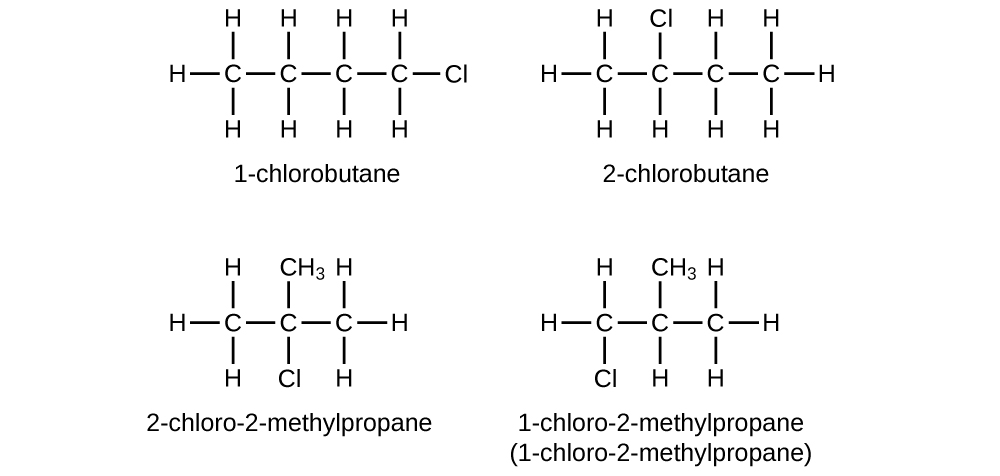


E Organic Chemistry Exercises Chemistry Libretexts
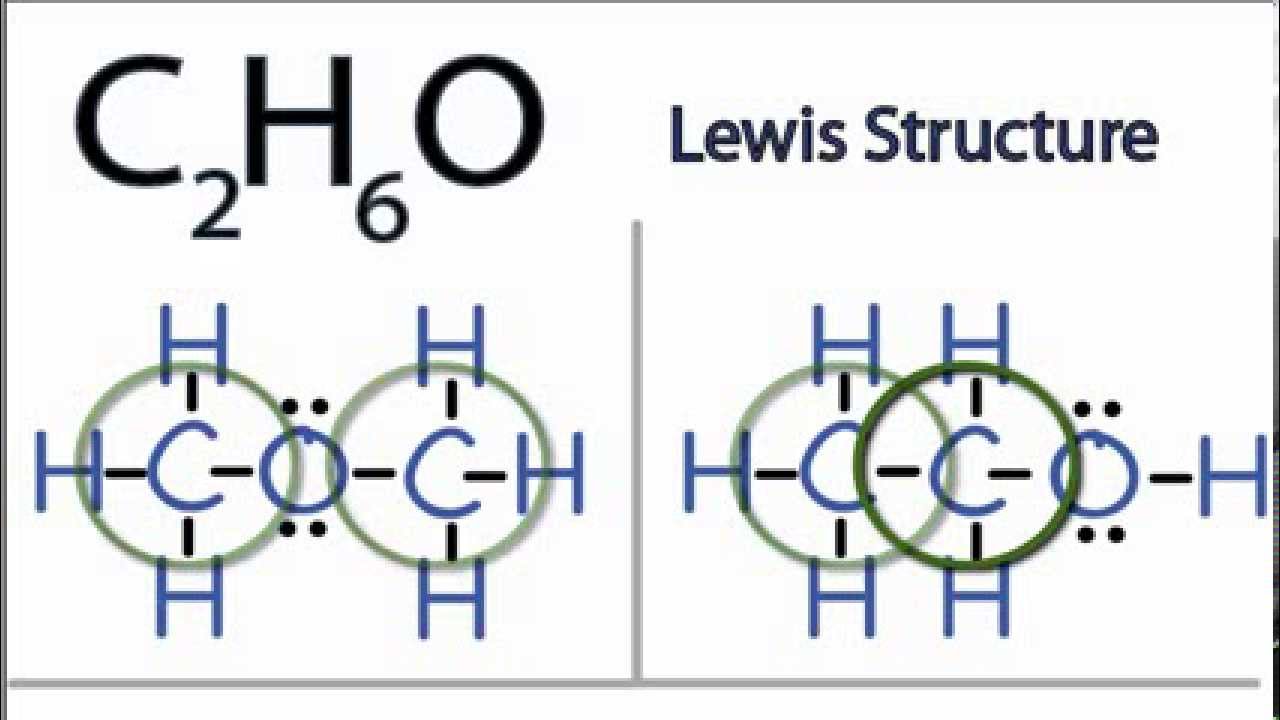


C2h6o Lewis Structure How To Draw The Lewis Structure For C2h6o Youtube
THERMOCHEMICAL INSIGHT INTO "GREEN CHEMISTRY" PROCESSES EXPERIMENT AND AB INITIO CALCULATIONS A THESIS submitted for the Degree of Doctor of PhilosophyIn the Lewis structures listed below, M and X represent various elements in the third period of the periodic table Write the formula of each compound using the chemical symbols of each element Write the Lewis structure for the diatomic molecule P 2, an unstable form of phosphorus found in hightemperature phosphorus vaporEthanol, CH 3 CH 2 OH;



Shortcuttochemistry April 11



Meso Hydrobenzoin 99 579 43 1 Sigma Aldrich
In a similar sense, the two Lewis structures for the SO 2 molecule are in resonance They mix to give a hybrid that is more than the sum of its components The fact that SO 2 is a resonance hybrid of two Lewis structures is indicated by writing a doubleheaded arrow between these Lewis structures, as shown in the figure above
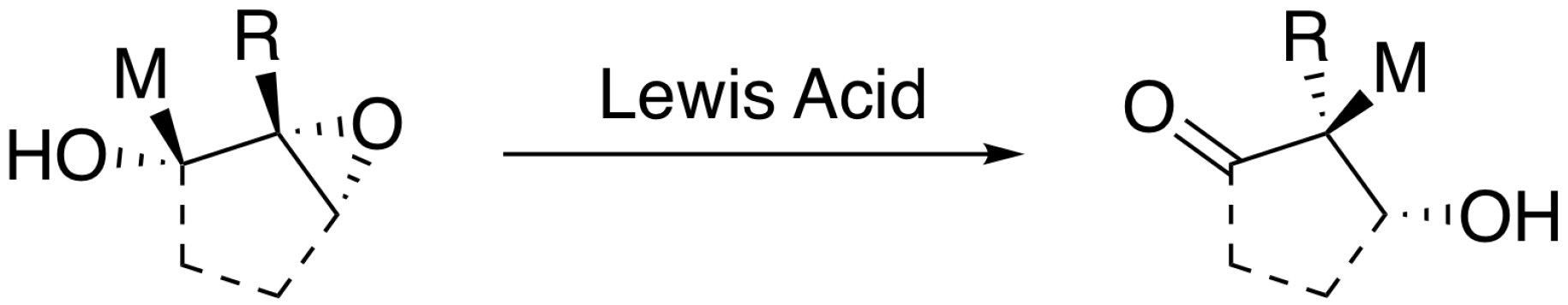


Molecules Free Full Text Cis Trans Energetics In Epoxide Thiirane Aziridine And Phosphirane Containing Cyclopentanols Effects Of Intramolecular Oh O S N And P Contacts Html
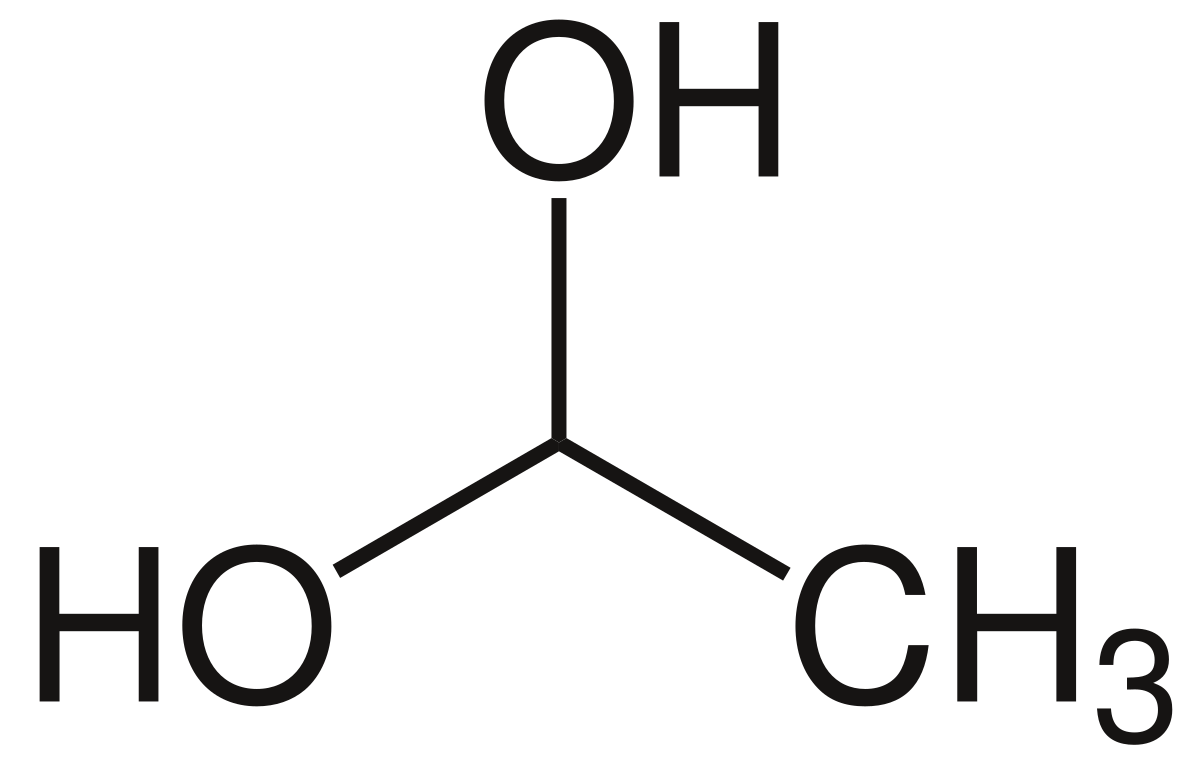


File 1 1 Ethanediol Svg Wikipedia


E Organic Chemistry Exercises Chemistry Libretexts
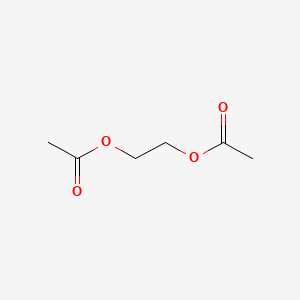


Ethylene Glycol Diacetate C6h10o4 Pubchem
.jpg)


Ethane 1 2 Diol Ethylene Glycol
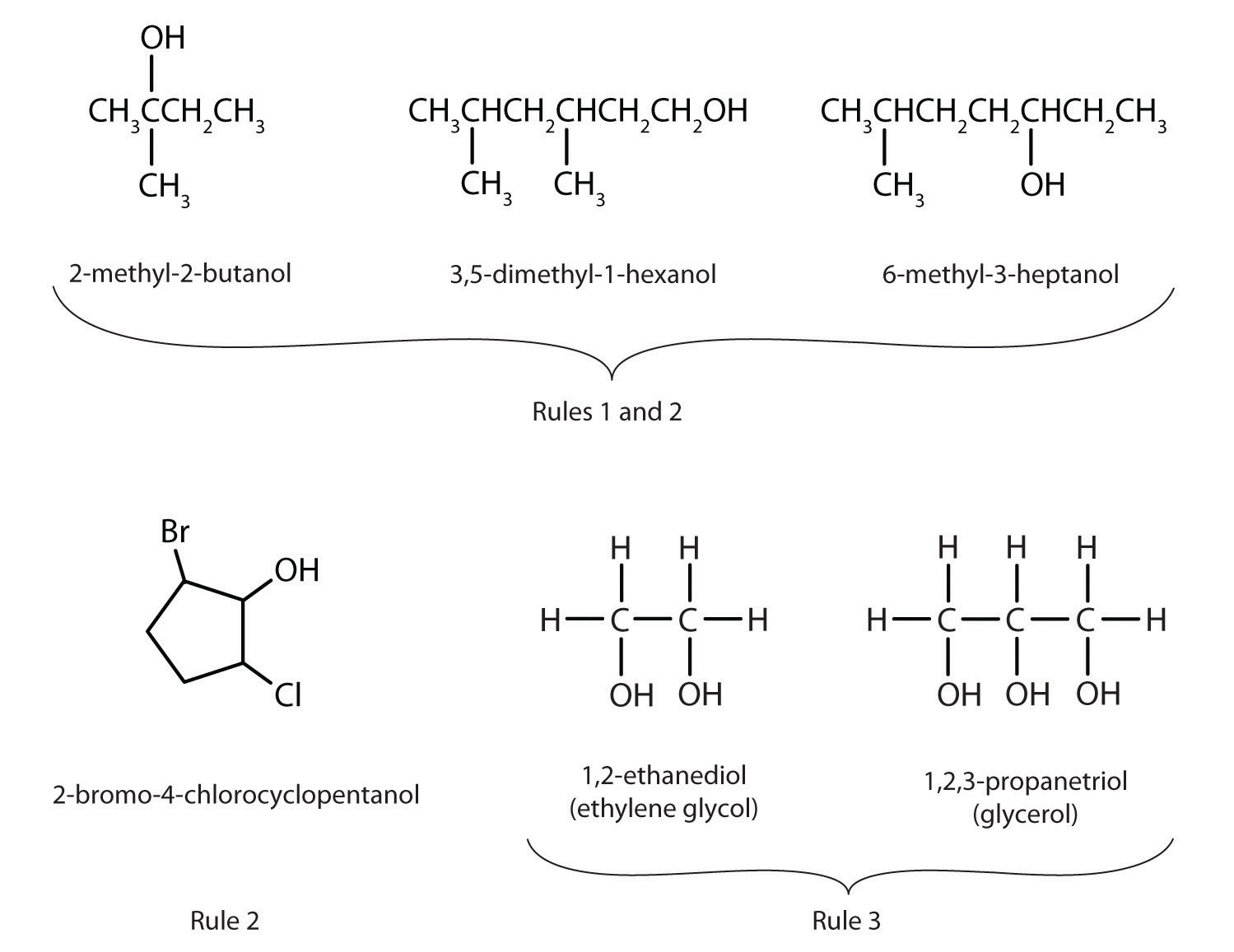


Alcohols Nomenclature And Classification
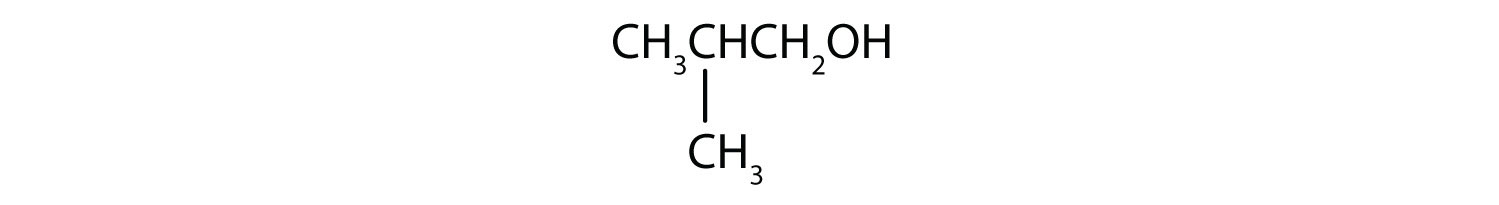


Alcohols Nomenclature And Classification


1 1 Ethanediol C2h6o2 Chemspider
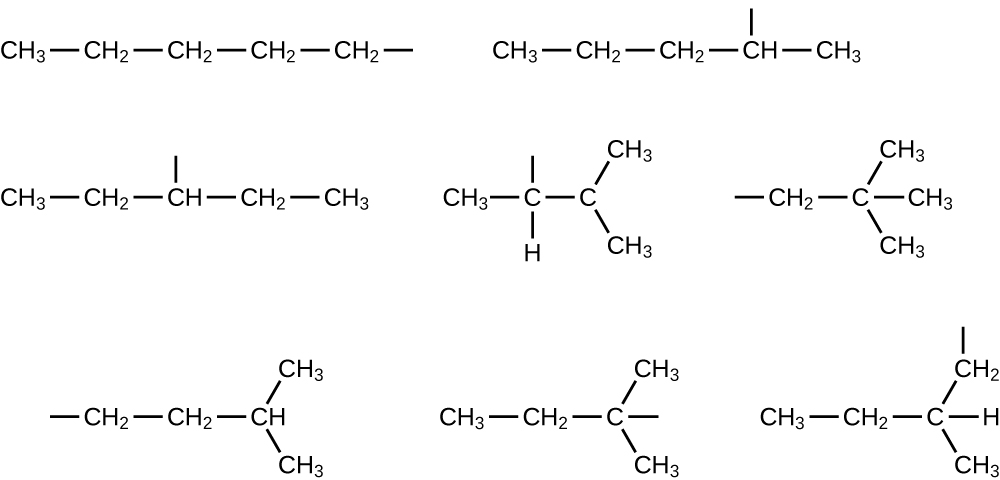


E Organic Chemistry Exercises Chemistry Libretexts



Meso Hydrobenzoin 99 579 43 1 Sigma Aldrich



4 Build A Model Of The 1 2 Ethanediol Molecule A Identify The Electron Pair And Molecular Geometry Around Homeworklib


Pushing Curly Arrows



Oneclass C Does The Molecule Have Only One Unique Shape In 3 D Hint How Are The Hydrogen Atoms O



See The Appendix For Information On How To Accurately Weigh Samples Using Weigh Course Hero
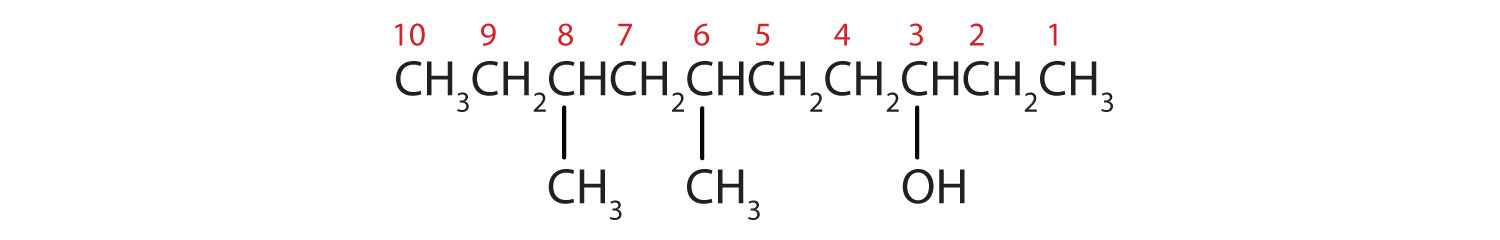


Alcohols Nomenclature And Classification


Pushing Curly Arrows
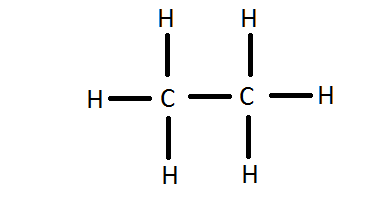


How Can I Write The Lewis Dot Structure For C2h6 Socratic



Emulsifiers Smoothex G M S Glyceryl Mono Stearate प यस क र Fine Zeelandia Private Limited Mumbai Id



Alcohols And Ethers Chemistry For Majors Atoms First



1 1 Ethanediol Structure C2h6o2 Over 100 Million Chemical Compounds Mol Instincts



Rcsb Pdb 3uet Crystal Structure Of Alpha 1 3 4 Fucosidase From Bifidobacterium Longum Subsp Infantis D172a E217a Mutant Complexed With Lacto N Fucopentaose Ii



Openstax General Chemistry Ch Organic Chemistry Top Hat



Propane 1 2 Diol An Overview Sciencedirect Topics



Alcohols And Ethers Chemistry For Majors Atoms First



Alcohols And Ethers Chemistry For Majors Atoms First



Organic Chemistry I Homework 1998
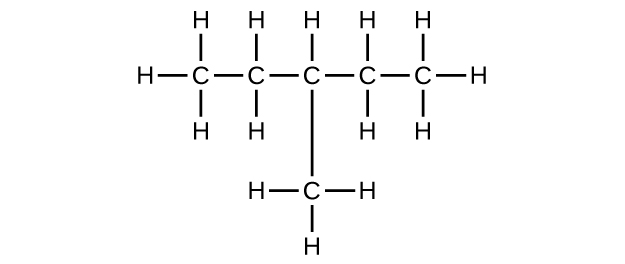


E Organic Chemistry Exercises Chemistry Libretexts



Draw Both The Condensed And The Structural Formula For Each Of The Following Compounds A 2 2 3 Trimethylpentane B 1 2 Ethanediol C 5 Methyl Trans 2 Heptene Study Com


Ethylene Glycol Molecule Of The Month June 18 Jsmol Version



Solved Chem 234 Spring 18 Pre Lab Worksheet Cyclic Ace Chegg Com



1 2 Ethanediol 1 4 Pyridinyl 1s 72 2 Wiki



Solved Organic Chemistry Due November 2 17 Name 1 4p Chegg Com



Ch3ch2oh Lewis Structure How To Draw The Lewis Structure For Ch3ch2oh Youtube



Propane 1 2 Diol An Overview Sciencedirect Topics


Chloral Hydrate C2h3cl3o2 Chemspider
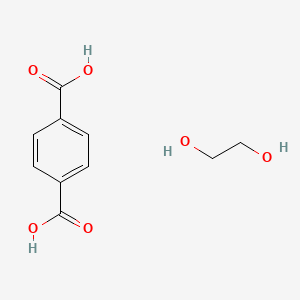


Ethane 1 2 Diol Terephthalic Acid C10h12o6 Pubchem



Solved 7 Ethylene Glycol 1 2 Ethanediol Has The Follow Chegg Com



1 2 4 Butanetriol An Overview Sciencedirect Topics
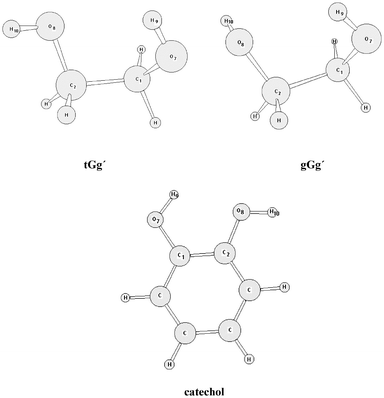


Do 1 2 Ethanediol And 1 2 Dihydroxybenzene Present Intramolecular Hydrogen Bond Physical Chemistry Chemical Physics Rsc Publishing


Pushing Curly Arrows



Alcohols And Ethers Chemistry For Majors Atoms First



Interpreting Condensed Chemical Structures Aleksmeaghane Troy4 25 Meaghane Troy4 25 pmest Course Hero


Glycolic Acid C2h4o3 Chemspider



Alcohols And Ethers Chemistry For Majors Atoms First
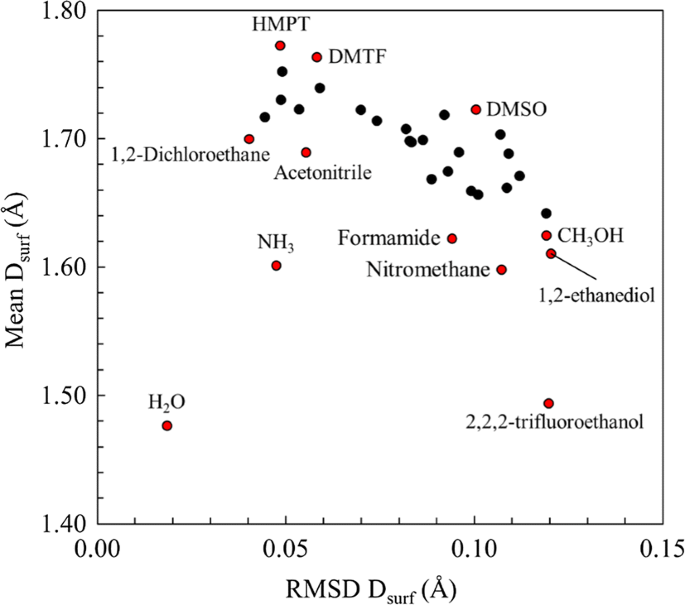


Extending The Marcus M Scale Of Solvent Softness Using Conceptual Density Functional Theory And The Orbital Overlap Distance Method And Application To Ionic Liquids Springerlink



1h Nmr Spectra Of Ethane 1 2 Diol And Other Vicinal Diols In Benzene Giao Dft Shift Calculations Lomas 13 Magnetic Resonance In Chemistry Wiley Online Library



Solved Change 1 2 Ethanediol Into 1 2 Ethenediol By Remov Chegg Com
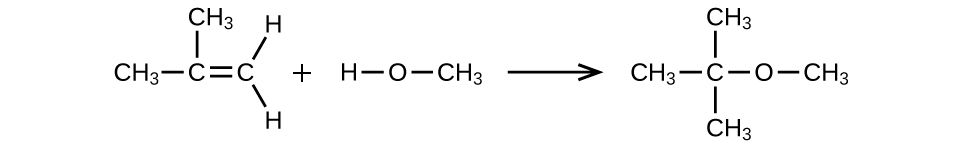


Alcohols And Ethers Chemistry 2e
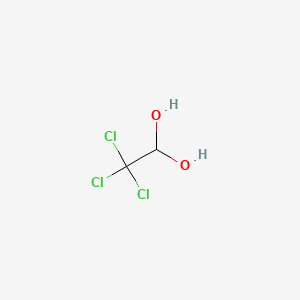


Chloral Hydrate C2h3cl3o2 Pubchem



Ethylene Glycol Properties Uses Structure Britannica



Environment And Climate Change Canada Acts Regulations Ethylene Glycol Final Content



Solved Can Somebody Draw The Mechanism For B C E And Expl Chegg Com



0 件のコメント:
コメントを投稿